NHS England North West GMSA - IHE Laboratory Testing Workflow (LTW)
0.0.1 - ci-build
NHS England North West GMSA - IHE Laboratory Testing Workflow (LTW)
0.0.1 - ci-build
DRAFT Implementation Guide
This is for collaboration and discussion purposes and is subject to change.
NHS England North West GMSA - IHE Laboratory Testing Workflow (LTW) - Local Development build (v0.0.1) built by the FHIR (HL7® FHIR® Standard) Build Tools. See the Directory of published versions
A. IHE Pathology and Laboratory Medicine (PaLM) Technical Framework - Volume 1
| Actor | Definition |
|---|---|
| Order Placer | Commonly known as the Electronic Patient Record (EPR) System or Order Communications System |
| Order Filler | Genomic Laboratory Hub (GLH), Laboratory Information System (LIMS) |
| Automation Manager | Performed by Laboratory Information System (LIMS) |
| Order Result Tracker | This is often provided by Electronic Patient Record (EPR) Systems |
| Laboratory Report (Clinical Document) | See Clinical Document |
| Intermediary | E.g. Regional or Trust Integration Engine |
See also Ref A Section 3 Laboratory Testing Workflow (LTW) Profile for detailed description of actors.
IHE LTW Actor Diagram
Initially only the IHE LAB-1 and LAB-4 is in focus.
Later stages will include the use of Genomic Order Management Service.
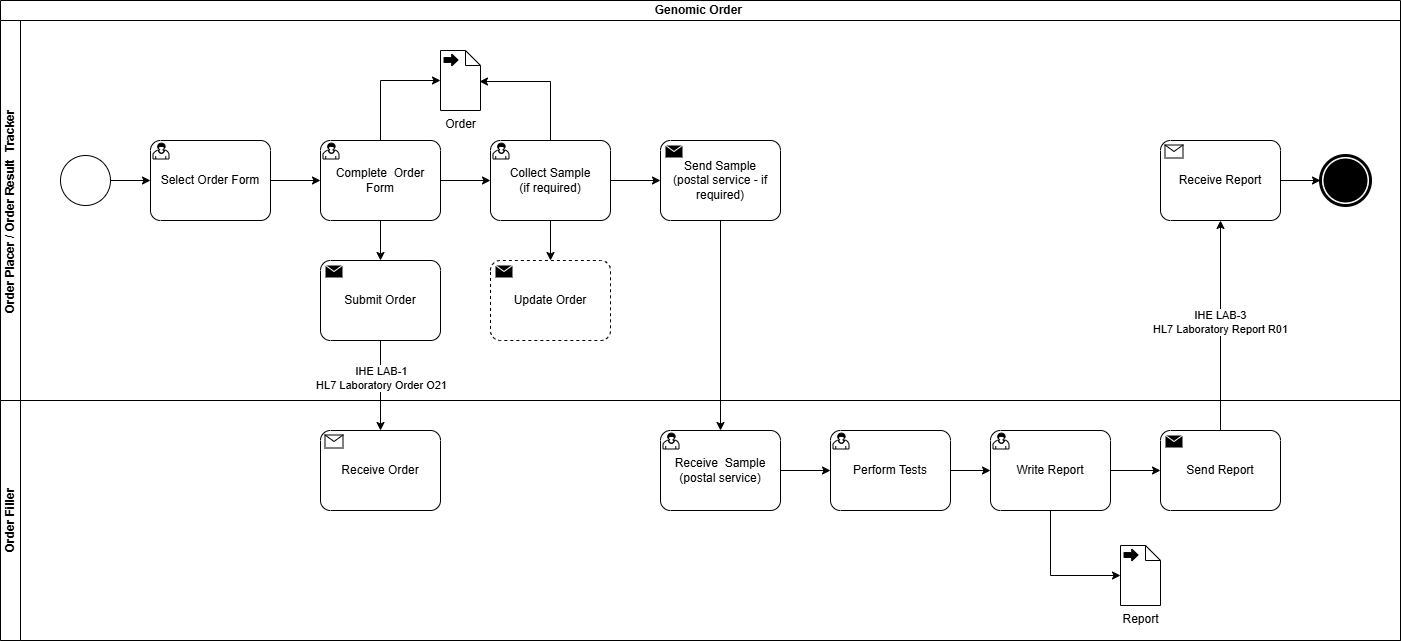
Genomic LTW Business Process
The sample may not need collecting by the ordering clinician for 2 reasons
- it has already been sent to the GLH and extracted DNA is already stored there
- the sample is somewhere else in the country. In this instance the ordering clinician will need to arrange the sample transfer to the GLH.
The processes above are described in more detail in:
From a high level perspective the process is
Genomics Simplified Sequence Diagram
Where the Order Placer sends the Laboratory Order to the Order Filler, the lab performs the test and then sends the Laboratory Report back to the Order Placer. However, variations can exist such as the order is updated or the order is entered directly on the Order Fillersystem (these are currently out of scope).
An order is created by the clinical practice, and placed to the laboratory.
Genomics Test Order Activity
Within the system creating the genomics order, the practitioner will select a form for the test required. Below are several examples from North West Genomic Laboratory Hub - Test Request Forms. How this is implemented will vary between different NHS organisations and systems they use.
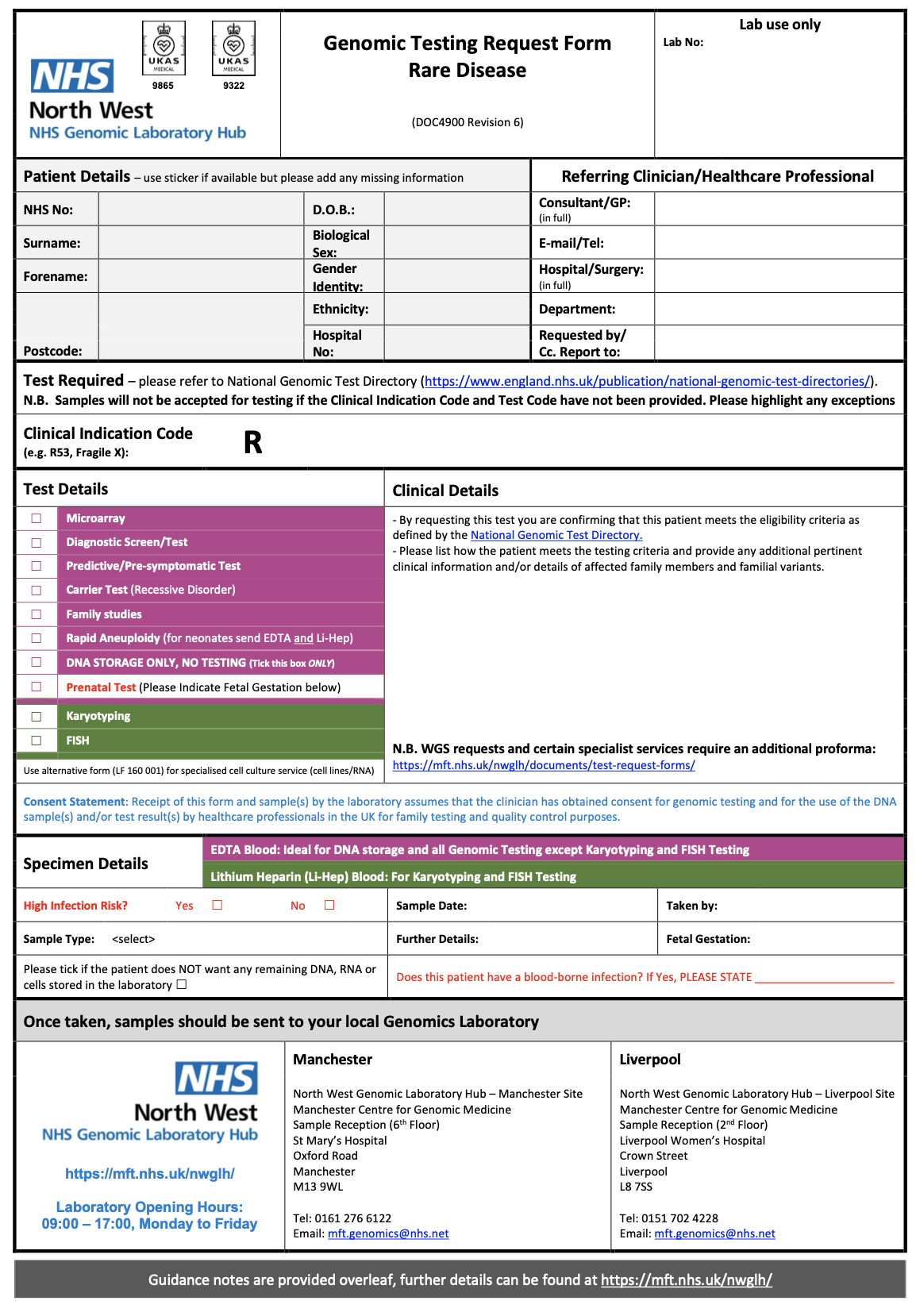
NW GLH Genomic Testing Request Form – Rare Disease |

Request for Genetic Testing for Haemoglobinopathies |
These forms may (/will?) will have a computable definition called an template (FHIR Questionnaire) which will list the technical content requirements for the form. At present only one archetype has been defined:
This archetype definition can also support HL7 Structured Data Capture should the Order Placer system support these features.
The completed form is submitted to the Regional Integration Engine using:
Genomics Test Order Sequence Diagram - LAB-1
For submission, this form will be converted by the Order Placer to a communication format called HL7 FHIR (and for compatability reasons HL7 v2. If the Order Placer has a FHIR enabled Electronic Patient Record (e.g. EPIC, Cerner, Meditech, etc), they may use HL7 SDC - Form Data Extraction to assist with this process.
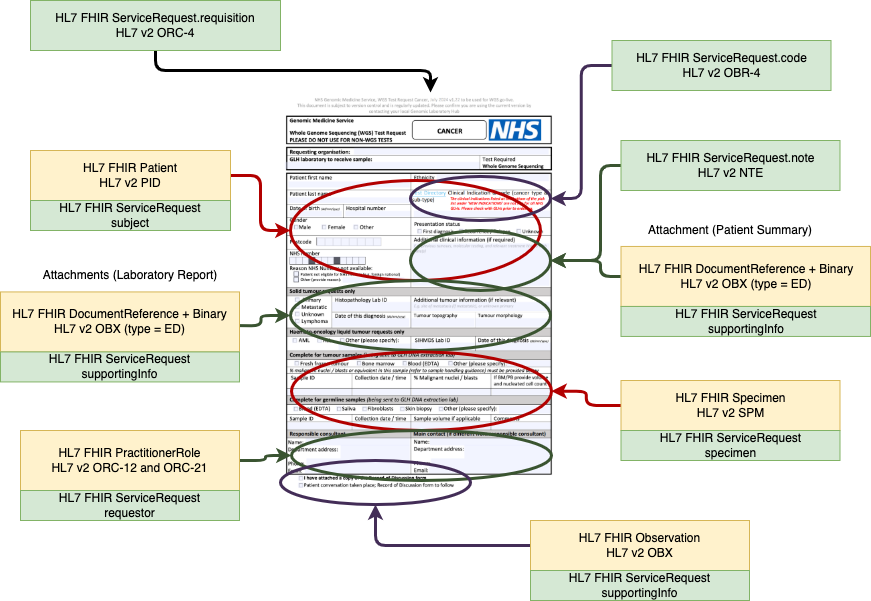
Order Test Form - Data Extraction Overview
The FHIR exchange style used FHIR Message following laboratory-order message definition. This definition is based on HL7 v2 OML_O21 Laboratory Order which simplifies conversion to/from pipe+hat (v2) and json (FHIR) formats.
At present the NW GLH Laboratory Information Management System (LIMS) will not support HL7 FHIR. The Regional Integration Exchange (RIE) will perform conversion between v2 and FHIR formats.
This message is an aggregate (DDD)/archetype and so is a collection of FHIR Resources (similar to v2 segements) which is described in Genomic Test Request Entity Model.
See also HL7 Europe Laboratory Report - ServiceRequest
This message can be extended by template (FHIR Questionnaire) which allows the definition of additional questions to be defined for the laboratory order.
The detail of this form/template defines:
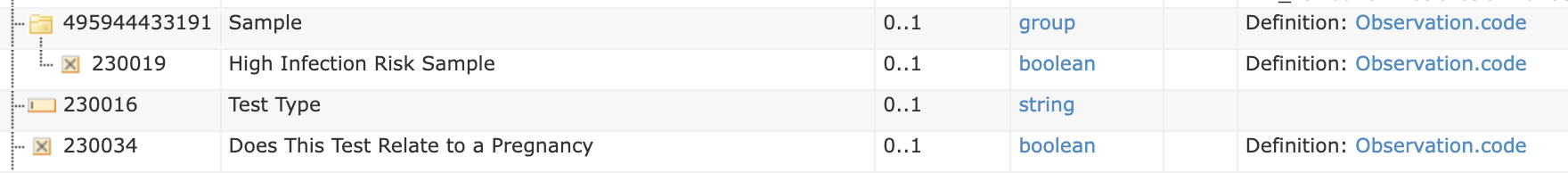
Order Text Form Example (extract)
| Question | CodeSystem | Code | FHIR Profile | HL7 v2 Segment | FHIR Questionniare item.type |
FHIR Observation value[x] |
v2 OBX-2 |
|---|---|---|---|---|---|---|---|
| Does This Test Relate to a Pregnancy | SNOMED | 77386006 | Observation | OBX | boolean | valueBoolean | CE (code 0136) |
| Sample | LOINC | 68992-7 | Observation-Panel | OBR | |||
| High Infection Risk Sample | SNOMED | 281269004 | Observation | OBX | boolean | valueBoolean | CE (code 0136) |
It is not expected the NW GLH Laboratory Information Management System (LIMS) will support UK SNOMED CT, and the RIE will handle the conversion either internally using FHIR ConceptMap or a terminology service with the following capabilities IHE Sharing Valuesets, Codes, and Maps (SVCM)
After submitting the original order, the sample will be collected and sent to the Order Filler. The Order Filler will update the Test Order to include details such as a specimen collection date, order filler number, etc.
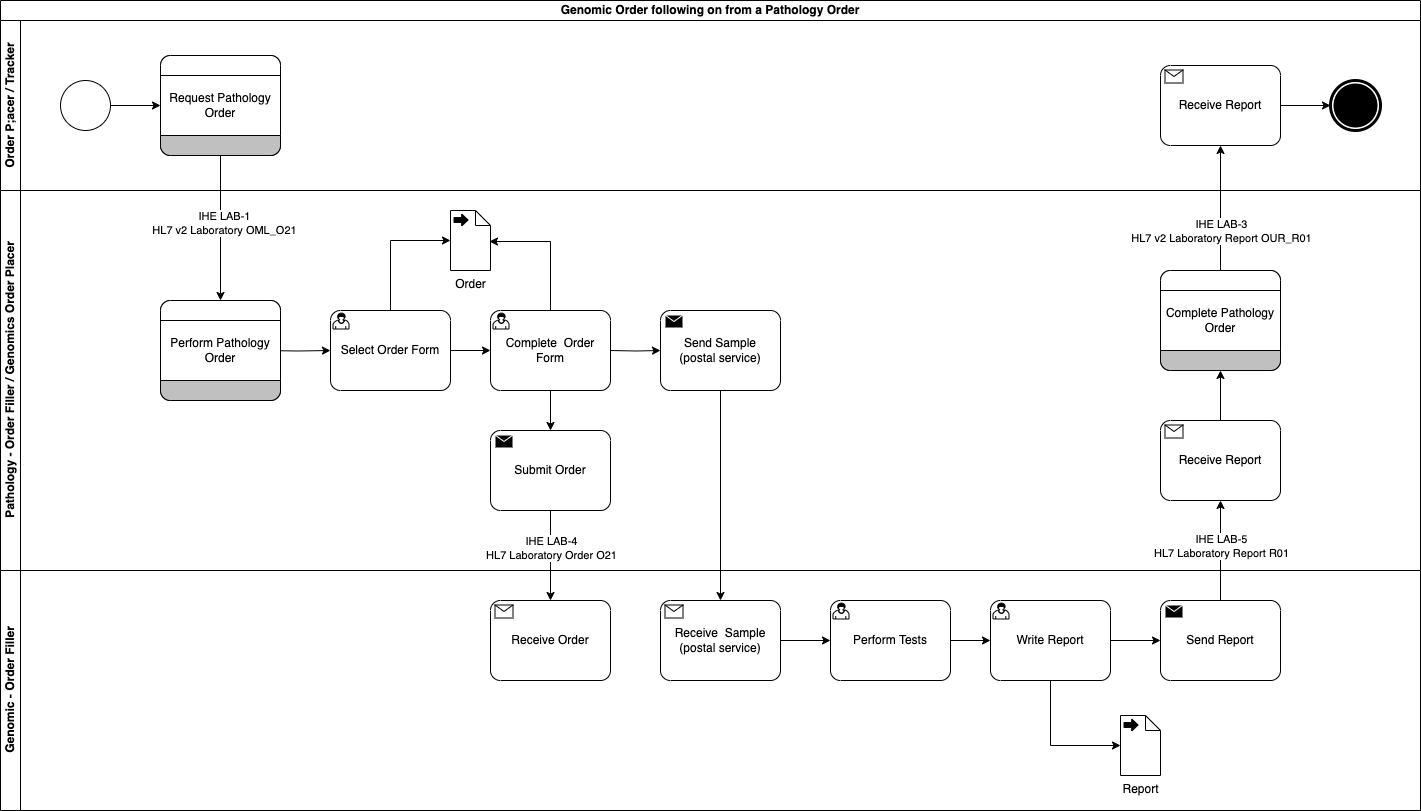
Genomic LTW Business Process - Use Case 3
In this use case the original order is raised by the Order Placer and sent to a Pathology LIMS (Pathology Order Filler). The Pathology LIMS follows the processes outlined in Use Case 1: Genomic Test Order and Use Case 4: Genomic Test Report for pathology testing.
As part of this testing, the clinical process requires a genomics test to be performed.
This genomics process is largely the same except for:
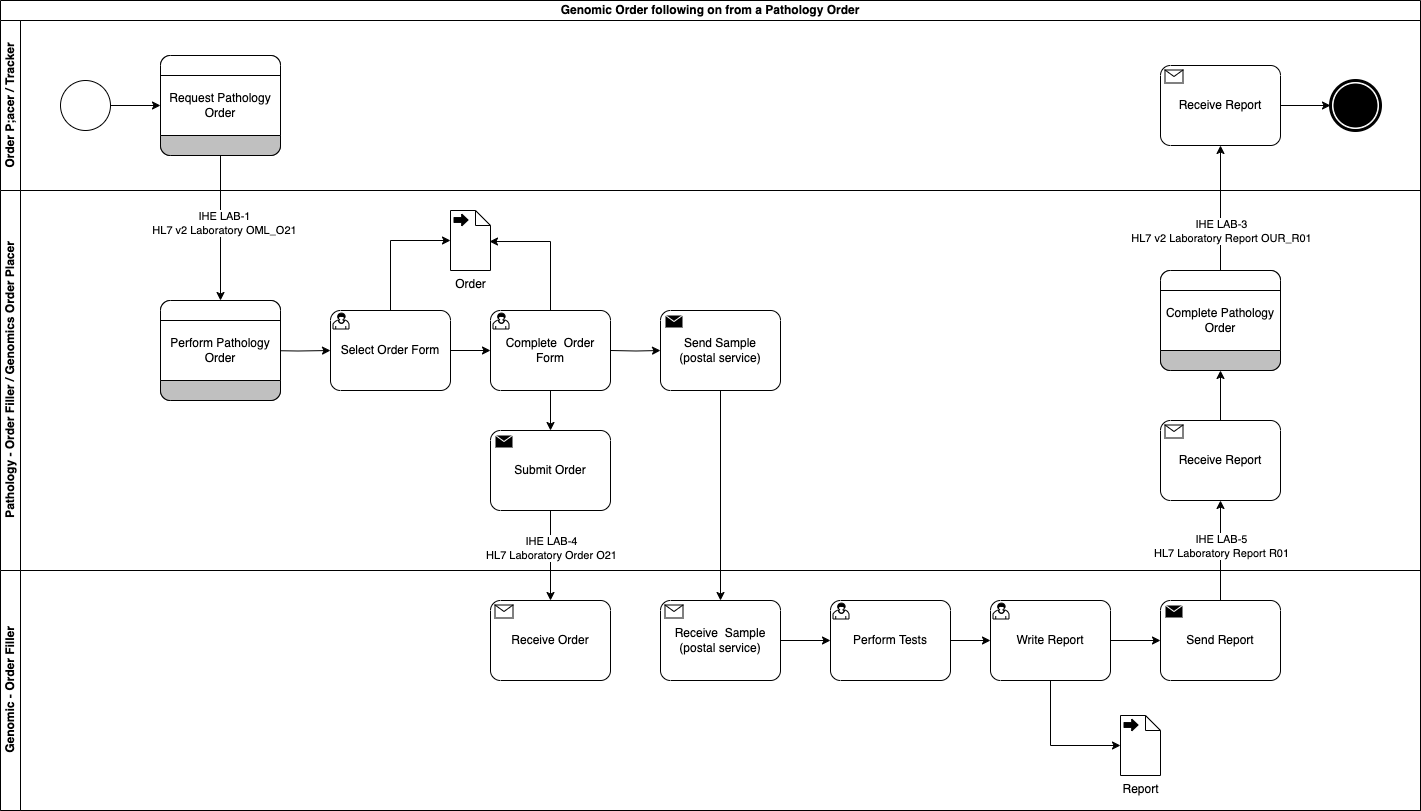
Genomic LTW Business Process - Use Case 4
In this use case the order has been manually entered into NW GLH LIMS (as a result of an email or telephone call).
The Order Filler notifies the Order Placer of the order.
The Order Placer can then update the Order Filler when details change on the order such as a Order Placer Number assigned or updating details on the specimen such as collection dates.
The proces then follows the same process as Use Case 4: Genomic Test Report
A report is created by the clinical practice, and sent to the order result tracker.
Genomics Test Report Activity
This guide implements the same use cases described in NHS England Pathology FHIR Implementation Guide - Background, with additions to support a wider set of actors and introduces standards around the Laboratory Order LAB-1. Key differences:
GP Electronic Patient Record System and Order Communications System are collectively known as Order Placer, this actor can also be fulfilled by other EPR systesm.GP Electronic Patient Record System is classed a Order Result Tracker, other systems may be used to deliver this functionality.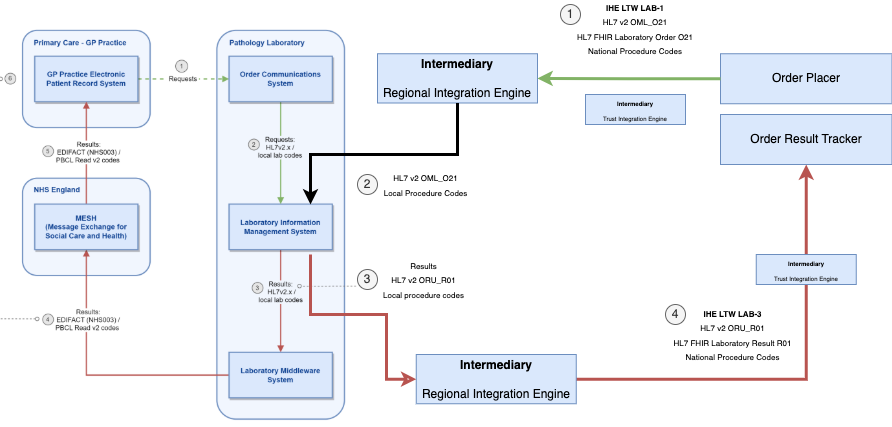
Relationship to NHS England Pathology
TODO
This may include IHE Internet User Authentication [IUA] and IHE Basic Audit Log Patterns[BALP] which includes use of:
It is recommended that the actors receive patient demographic and encounter updates only within the context of a work order. Whenever patient data changes, due to:
Note: Event trigger definitions based on NHS England HL7 v2 ADT Message Specification which is NHS England’s supplement to IHE Technical Framework Volume2: Patient Identity Management [ITI-30] and Patient Encounter Management [ITI-31].
It is common for this requirement to be answered by a combination of: